2021.12.03
RIKEN Immune Regenerative Medicine Inc. was introduced at an FEC Europe Executive Meeting that was co-sponsored by the French Ambassador to Japan, Philippe Seton, and the International Friendship Exchange Council (FEC).
On October 6, 2021 Jie Tokuoka, CEO of RIKEN Immune Regenerative Medicine Inc., a company that develops NKT cell-targeted therapy "RIKNKT®", gave a lecture at the Europe Executive Meeting sponsored by the International Friendship Exchange Council (FEC) in front of the Ambassador of France to Japan, Philippe Seton.
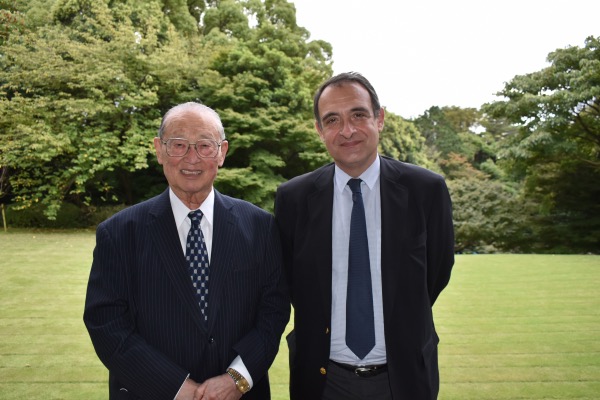

RIKEN Immune Regenerative Medicine Inc. (Representative Director: Jie Tokuoka, Headquarters: 10-2 Ichibancho, Chiyoda-ku, Tokyo) has improved and put into practical use the NKT cell-targeted therapy that RIKEN and Chiba University have been conducting clinical trials, is the developer of the new NKT cell-targeted treatment “RIKNKT®”.Currently, we are providing regenerative medicine products to 18 medical institutions nationwide.On October 6, 2021, the company's representative, Jie Tokuoka, attended the FEC the Europe Executive Meeting co-hosted by the FEC held at the Embassy of the French Republic in Japan with Philippe Seton, the French Ambassador to Japan, in attendance. He gave a lecture on the company's business and technology and deepened ties with local officials.The executive meeting was attended by Philippe Seton, the French Ambassador to Japan, embassy officials, and Mr. Ken Matsuzawa, President of the FEC. Mr. Seton, the French Ambassador to Japan, made a presentation focusing on the current socio-economic culture of the French Republic and especially its economic policies.The purpose of this executive meeting is to deepen mutual understanding between Japan and the French Republic and to provide a forum for exploring the possibility of concrete business developments for excellent companies.
The executive meeting was attended by 23 people, including officials from the French Embassy in Japan, the media, and managers from various fields. After the messages were delivered by Ambassador Philippe Seton and Mr. Ken Matsuzawa, Ambassador Philippe Seton gave a presentation focusing on the Republic's economic policies, measures to attract foreign companies, and the introduction of new technologies.Afterward, the Japanese participants gave presentations on their companies and their technologies, and at the end, there was an opportunity to chat. In the future, there are plans to hold concrete discussions regarding the deployment of the company's technology in the country.



RIKEN Immune Regenerative Medicine Inc. is a Japanese medical science company headquartered in Chiyoda-ku, Tokyo.The company name "RIKEN" is used because it is a company that originated from RIKEN.Established in 2014, the company developed RIKNKT ®, a cancer treatment technology using NKT cells, which are special immune cells, and provides regenerative medicine products for cancer treatment to medical institutions.
Riken Immune Regenerative Medicine http://www.riken-irm.com
FEC: An organization established in 1983 with the aim of deepening exchanges and mutual understanding through various projects, including economic, cultural, and personal exchanges with countries around the world at the private level.Based on the three basic principles of contributing to "world peace and prosperity," "response to the age of globalization," and "strongly promoting intergovernmental diplomacy," the Chairman is Chihiro Kanagawa, Chairman of Shin-Etsu Chemical Co., Ltd., Ken Matsuzawa, Advisor and former Chairman of NIPPONKOA Insurance Co., Ltd. as the chairman, mayors of designated cities, about 120 people are honorary members.
FEC the International Friendship Exchange Councilhttps://www.fec-ais.com
RIKNKT®: Further improvements to the medical technology "NKT cell-targeted therapy" for the treatment of cancer, which has been researched and developed over a long period of time by RIKEN, an independent development research agency, and has been clinically tested with Chiba University. It is a cancer treatment technology using autologous immune cells that was originally developed by RIKEN Immune Regenerative Medicine.Focusing on the properties of special immune cells, NKT cells, which are contained in the blood in extremely small amounts, it activates the NKT cells in the body.
Activated NKT cells attack cancer cells while restoring the body's immune environment through immune checkpoint inhibition and perforin effects, and cytokines produced by activated NKT cells strongly activate innate immune system NK cells and adaptive immune system T cells, and these cells also attack cancer cells.It has also been shown that activated NKT cells acquire long-term immunological memory.
Due to its unique mechanism, RIKNKT® is applicable to all people, almost all cancer types and sites, and all stages from stage I to IV.In addition, since it is a treatment method that uses autologous immune cells, there are almost no side effects.In particular, it is expected to be effective in preventing recurrence and metastasis before and after surgery, and it can be used in combination with standard anticancer drugs and radiotherapy.
The essential ligand (α-galactosylceramide) that activates NKT cells in the body, which is used in RIKNKT®, was independently synthesized by RIKEN Immune Regenerative Medicine, and it is manufactured in compliance with GMP through a contract with a domestic pharmaceutical manufacturer. *2, and RIKEN Immune Regenerative Medicine holds exclusive rights to its manufacture and supply.
RIKNKT® has been approved by the Ministry of Health, Labor and Welfare since June 2016 and has a track record of more than 200 cases in Japan to date in which cancer progression was halted or in complete remission, with high success rates of no recurrences, were observed in patients who had undergone treatment, regardless of the site or stage of cancer, and a remarkable improvement in QOL, including a reduction in the side effects of anticancer drugs, has been confirmed.
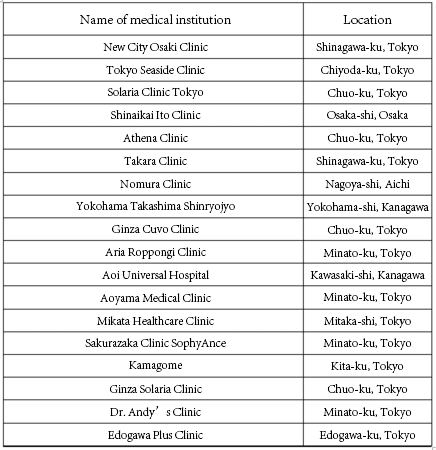
Currently, we are providing RIKNKT® regenerative medicine products to 18 carefully selected affiliated medical institutions*3 nationwide.The treatment is not covered by insurance, and the treatment cost is about 3.52 million yen (including tax)*3.
※1 A unique synthesis method was invented by RIKEN Immune Regenerative Medicine Inc., and it is manufactured in compliance with GMP through a contract with domestic pharmaceutical manufacturer.GMP (Good Manufacturing Practice) refers to "good manufacturing practices" (manufacturing control and quality control standards) required of manufacturers (including foreign manufacturers) and marketing authorization holders. Quality control refers to work related to the receipt of raw materials such as pharmaceuticals, inspection, manufacturing, product packaging, shipping management, product storage, collection processing, etc.Pharmaceutical manufacturing is stipulated to comply with the GMP ministerial ordinance based on the Pharmaceutical Affairs Law, which also applies to medical institutions that conduct clinical trials.
※2 List of medical institutions that provide RIKNKT® (in no particular order).
※3 The treatment cost of about 3.52 million yen (tax included) includes all costs such as the initial examination fee, pre-examination fee, re-examination fee, blood collection fee, administration fee, immune function test, etc. However, depending on the medical institution, there are some differences in treatment costs, such as transportation costs and examination costs.
For inquiries regarding this release, please contact RIKEN Immune Regenerative Medicine Inc. (representative number 03-5226-5880).




